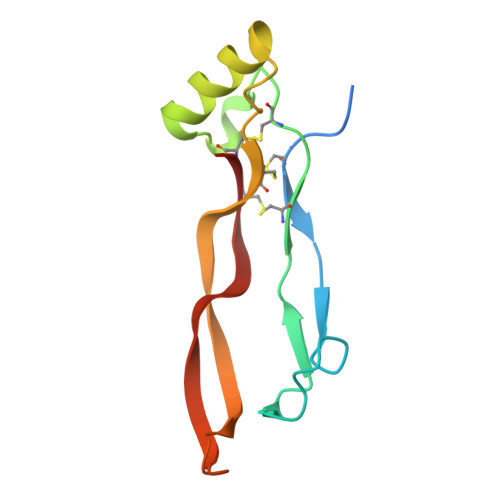
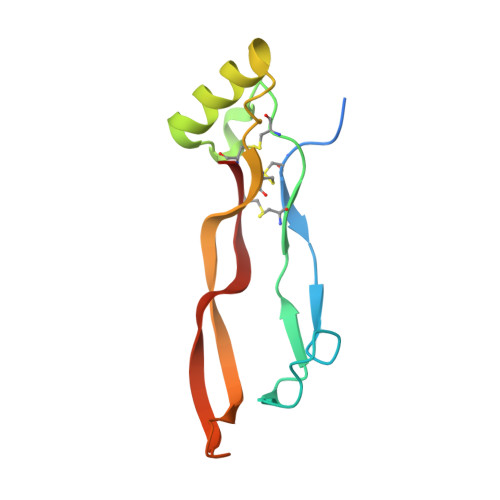
An Activin A/BMP2 Chimera, AB204, Displays Bone-Healing Properties Superior to Those of BMP2.
Yoon, B.H., Esquivies, L., Ahn, C., Gray, P.C., Ye, S.K., Kwiatkowski, W., Choe, S.(2014) J Bone Miner Res 29: 1950-1959
- PubMed: 24692083
- DOI: https://doi.org/10.1002/jbmr.2238
- Primary Citation of Related Structures:
4MID - PubMed Abstract:
Recombinant bone morphogenetic protein 2 (rhBMP2) has been used clinically to treat bone fractures in human patients. However, the high doses of rhBMP2 required for a therapeutic response can cause undesirable side effects. Here, we demonstrate that a novel Activin A/BMP2 (AB2) chimera, AB204, promotes osteogenesis and bone healing much more potently and effectively than rhBMP2. Remarkably, 1 month of AB204 treatment completely heals tibial and calvarial defects of critical size in mice at a concentration 10-fold lower than a dose of rhBMP2 that only partially heals the defect. We determine the structure of AB204 to 2.3 Å that reveals a distinct BMP2-like fold in which the Activin A sequence segments confer insensitivity to the BMP2 antagonist Noggin and an affinity for the Activin/BMP type II receptor ActRII that is 100-fold greater than that of BMP2. The structure also led to our identification of a single Activin A-derived amino acid residue, which, when mutated to the corresponding BMP2 residue, resulted in a significant increase in the affinity of AB204 for its type I receptor BMPRIa and a further enhancement in AB204's osteogenic potency. Together, these findings demonstrate that rationally designed AB2 chimeras can provide BMP2 substitutes with enhanced potency for treating non-union bone fractures.
Organizational Affiliation:
Protein Engineering Laboratory, Joint Center for Biosciences at Songdo Global University Campus, Incheon, Republic of Korea; Department of Pharmacology, Seoul National University College of Medicine, Seoul, Republic of Korea.