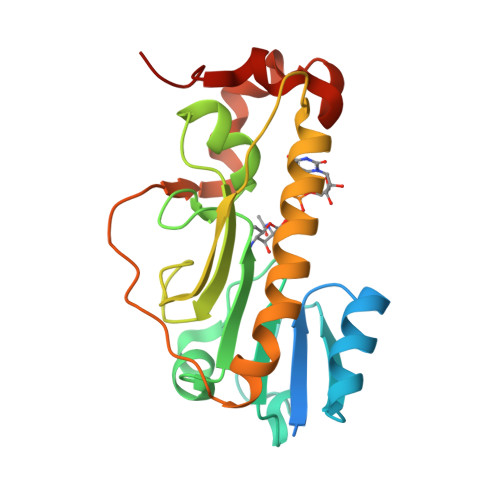
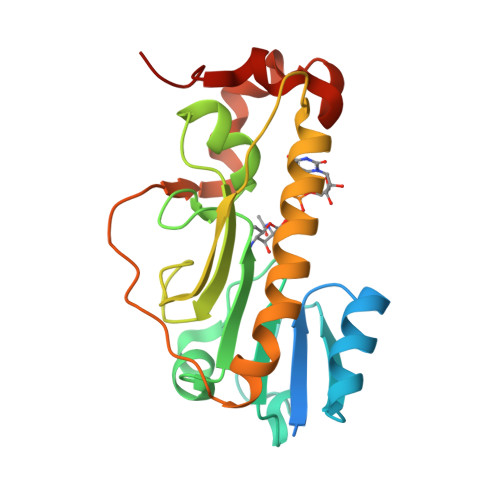
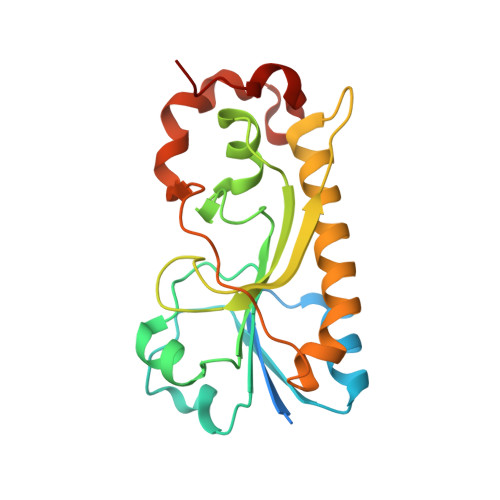
Structural Insight into a Novel Formyltransferase and Evolution to a Nonribosomal Peptide Synthetase Tailoring Domain.
Reimer, J.M., Harb, I., Ovchinnikova, O.G., Jiang, J., Whitfield, C., Schmeing, T.M.(2018) ACS Chem Biol 13: 3161-3172
- PubMed: 30346688
- DOI: https://doi.org/10.1021/acschembio.8b00739
- Primary Citation of Related Structures:
6CI2, 6CI4, 6CI5, 6EDK - PubMed Abstract:
Nonribosomal peptide synthetases (NRPSs) increase the chemical diversity of their products by acquiring tailoring domains. Linear gramicidin synthetase starts with a tailoring formylation (F) domain, which likely originated from a sugar formyltransferase (FT) gene. Here, we present studies on an Anoxybacillus kamchatkensis sugar FT representative of the prehorizontal gene transfer FT. Gene cluster analysis reveals that this FT acts on a UDP-sugar in a novel pathway for synthesis of a 7-formamido derivative of CMP-pseudaminic acid. We recapitulate the pathway up to and including the formylation step in vitro, experimentally demonstrating the role of the FT. We also present X-ray crystal structures of the FT alone and with ligands, which unveil contrasts with other structurally characterized sugar FTs and show close structural similarity with the F domain. The structures reveal insights into the adaptations that were needed to co-opt and evolve a sugar FT into a functional and useful NRPS domain.
Organizational Affiliation:
Department of Biochemistry , McGill University , Montréal , Québec H3G 0B1 , Canada.