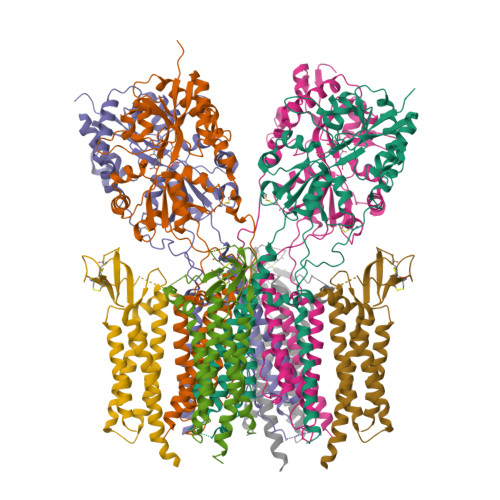
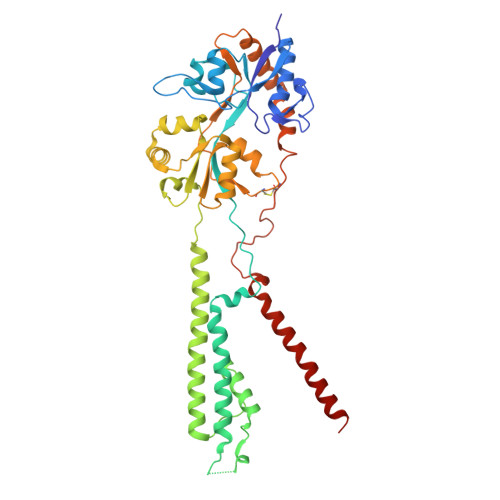
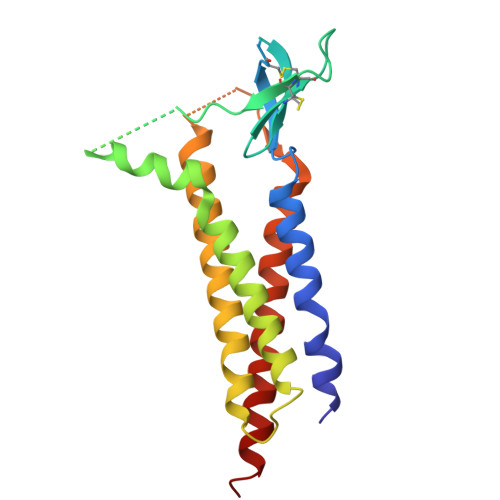
Glutamate gating of AMPA-subtype iGluRs at physiological temperatures.
Kumar Mondal, A., Carrillo, E., Jayaraman, V., Twomey, E.C.(2025) Nature
- PubMed: 40140570
- DOI: https://doi.org/10.1038/s41586-025-08770-0
- Primary Citation of Related Structures:
9DHP, 9DHQ, 9DHR, 9DHS, 9DHT, 9MRK, 9MRL, 9MRM, 9MRN - PubMed Abstract:
Ionotropic glutamate receptors (iGluRs) are tetrameric ligand-gated ion channels that mediate most excitatory neurotransmission 1 . iGluRs are gated by glutamate, where on glutamate binding, they open their ion channels to enable cation influx into postsynaptic neurons, initiating signal transduction 1,2 . The structural mechanics of how glutamate gating occurs in full-length iGluRs is not well understood. Here, using the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid subtype iGluR (AMPAR), we identify the glutamate-gating mechanism. AMPAR activation by glutamate is augmented at physiological temperatures. By preparing AMPARs for cryogenic-electron microscopy at these temperatures, we captured the glutamate-gating mechanism. Activation by glutamate initiates ion channel opening that involves all ion channel helices hinging away from the pore axis in a motif that is conserved across all iGluRs. Desensitization occurs when the local dimer pairs decouple and enables closure of the ion channel below through restoring the channel hinges and refolding the channel gate. Our findings define how glutamate gates iGluRs, provide foundations for therapeutic design and demonstrate how physiological temperatures can alter iGluR function.
Organizational Affiliation:
Department of Biophysics and Biophysical Chemistry, Johns Hopkins University School of Medicine, Baltimore, MD, USA.